PHDS is valued by clients for its achievement of developing SFDA-approved new drug;
PHDS is valued by clients for its achievement of developing SFDA-approved new drug;
 PHDS specializes in registration and regulatory affairs by registering medicines and health products in China and helps global clients bringing their novel products to China;
PHDS specializes in registration and regulatory affairs by registering medicines and health products in China and helps global clients bringing their novel products to China;
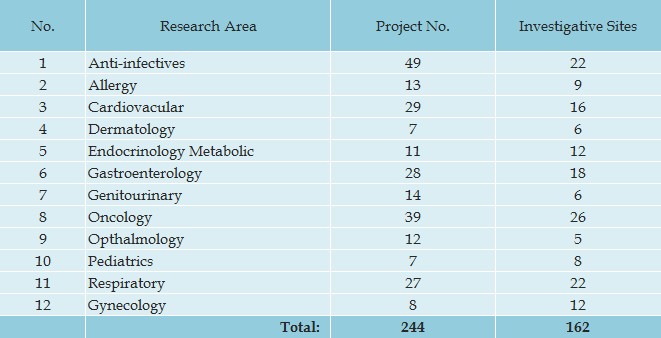
 PHDS is also dedicated to the management of global multi-center clinical trials. Over 12 therapeutic areas, PHDS stands out with impressive professionalism and outstanding results;
PHDS is also dedicated to the management of global multi-center clinical trials. Over 12 therapeutic areas, PHDS stands out with impressive professionalism and outstanding results;
 PHDS expertise and quality service are followed by abundant successful cases.
PHDS expertise and quality service are followed by abundant successful cases.
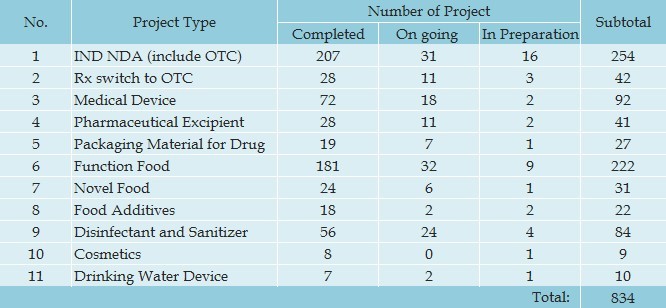
Regulatory Projects:
Our record shows that PHDS has successfully completed 834 projects at end of September 2012.
Clinical Trials:
Our record shows that PHDS has successfully completed 244 studies at end of September 2012.